Polymeric Microspheres for Sustained Release Delivery
The market trend is clear - microsphere-based "depot" products dominate, driving billions in annual sales. These products, often based on biodegradable PLGA microspheres, are administered subcutaneously or intramuscularly, offering convenient dosing frequencies ranging from once a week to once every several months.
Below are just some of the benefits of Sustained-release Drug Delivery systems:
- Improved patient compliance
- Reduced systemic exposure resulting in decreased side effects
- Targeting of anatomical areas
- Enhanced therapeutic effect
With over 15 years of expertise, Phosphorex stands as one of the leaders for developing particulate-based drug delivery systems for sustained release. Our focus on encapsulating peptides, proteins, and small molecules caters to the growing demand for advanced therapeutic solutions.
Partnering with clients, we leverage a diverse range of biodegradable polymers such as PLA, PCL, polyanhydrides, and poly(ortho esters). Together, we formulate solutions that achieve desired therapeutic effects, reduce side effects, and enhance patient compliance.
Phosphorex excels in sustained release particulate-based drug delivery across diverse therapeutic areas.
Our expertise covers:
- Peptides, proteins, and small molecules (hydrophilic and hydrophobic) as drug payloads
- Pioneering research applications in oncology, pulmonary, and ocular fields
What does partnering with Phosphorex look like?
Phosphorex offers seamless support from proof of concept to clinical trials. Our collaborative process ensures the development of microsphere systems that align with your product profile. We prioritize scalability and clinical relevance, facilitating a smooth transition to large-scale manufacturing.
Our comprehensive take on your project includes:
Formulation Feasibility:
-
Manufacture and characterization of small-scale batches, from milligrams to grams, to develop prototypes meeting the target product profile
-
Identification of lead candidates based on in vitro characterization for further testing in animal models
Process Development and Scale-Up:
-
Selection of a lead formulation based on feasibility and in vivo study results
-
Definition of critical quality attributes using the target product profile
-
Stepwise scale-up approach, focusing on unit operations and critical process parameters
-
Utilization of semi-continuous and continuous manufacturing processes for consistent formulation performance
-
Supply of pilot batch(es) for IND-enabling pre-clinical studies
Clinical Batch Manufacture:
-
GMP manufacturing capabilities available soon
-
Support for clinical manufacturing needs through our network of CMOs
Analytical Capabilities:
Formulation, process development, and analytics are interconnected. Advancing analytical method development in a stage appropriate manner is critical to ensure success of program from feasibility to clinical manufacture. Phosphorex’s analytical services for sustained release drug delivery systems include API characterization, physical characterization, and development stability studies.
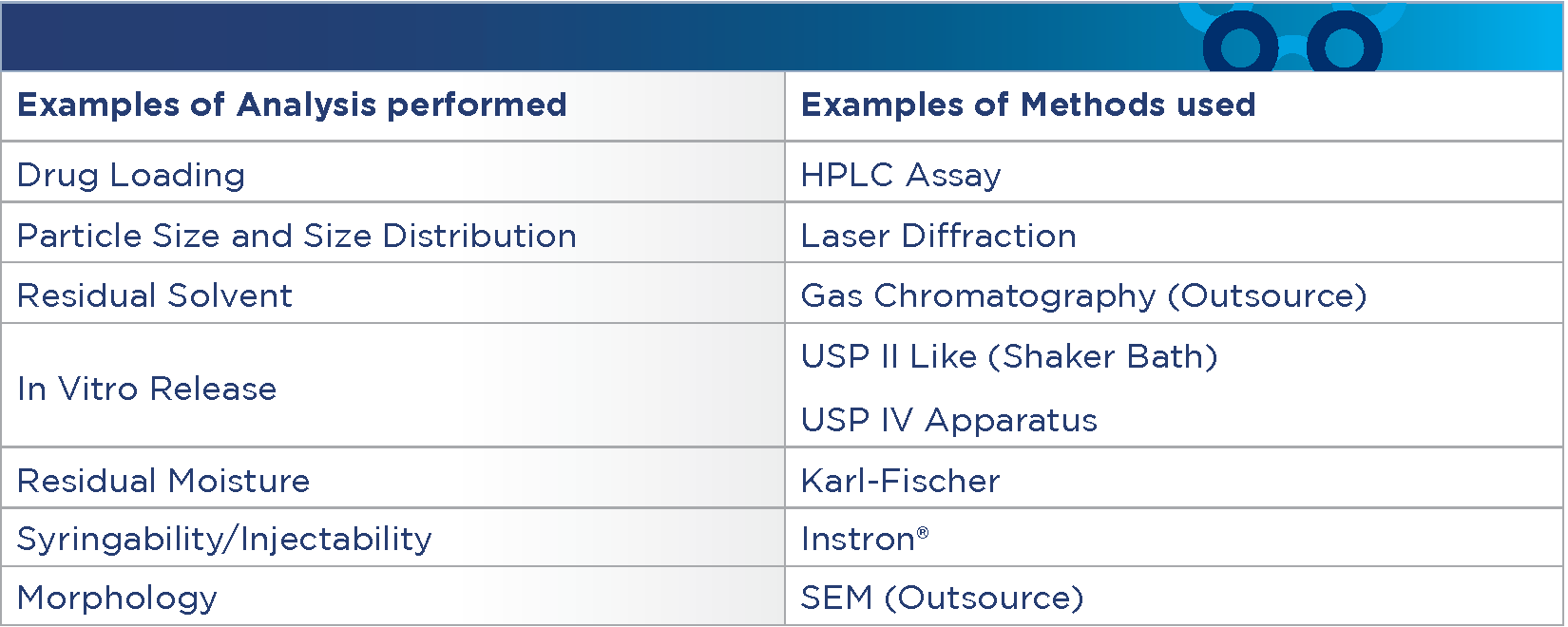
What differentiates us?
-
15+ years' experience in sustained release microsphere development
-
Flexible team of experts for formulation feasibility, process development, and clinical manufacturing (late 2024)
-
Comprehensive analytical capabilities, including USP II and USP IV in vitro release assays
-
Scalable and clinically relevant semi-continuous manufacturing processes
Leverage our expertise and capabilities to advance your sustained release microspheres development. Contact us for more info at bd@phosphorex.com