Process Development & Scale-up
Our Process Development team has a track record of excellence in scaling complex particle processes, such as Lipid Nanoparticles (LNPs), Polymeric Nanoparticles (PNPs), and Polymeric Microspheres (PMPs), from bench-scale to clinical-scale. Our success lies in our ability to utilize cGMP manufacturing-relevant unit operations and cutting-edge equipment.
Phosphorex’s Process Development Capabilities include:
- Scale-up of small and bench nanoparticle formulations to clinical-scale
- Production of material for IND enabling studies at the clinical-scale;
- Execution of a tech transfer of the scaled-up process to cGMP manufacturing
- In-process and final product testing
For Process Development, we provide the following services offerings:
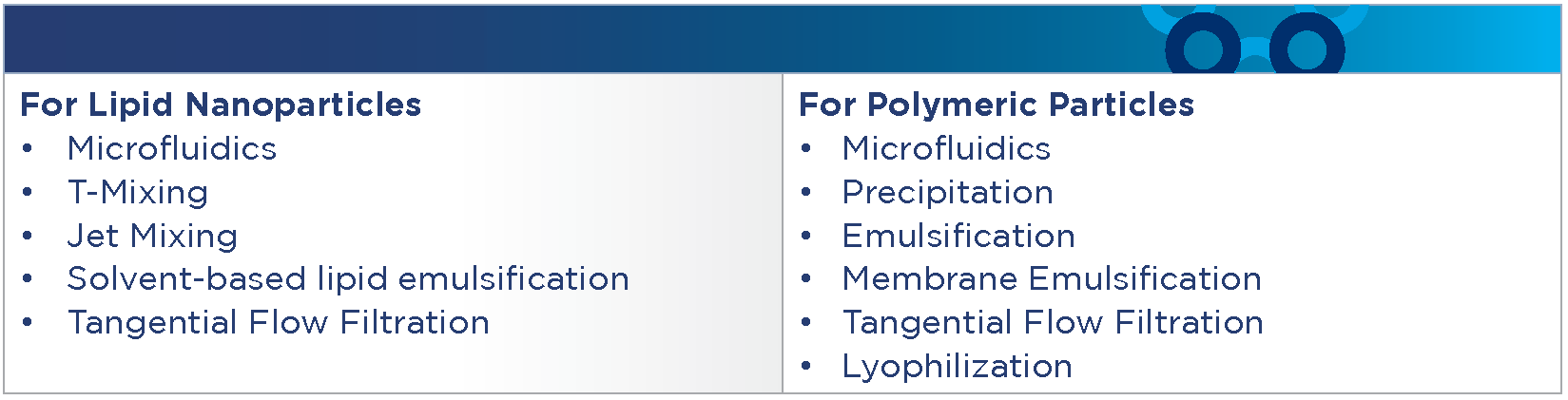
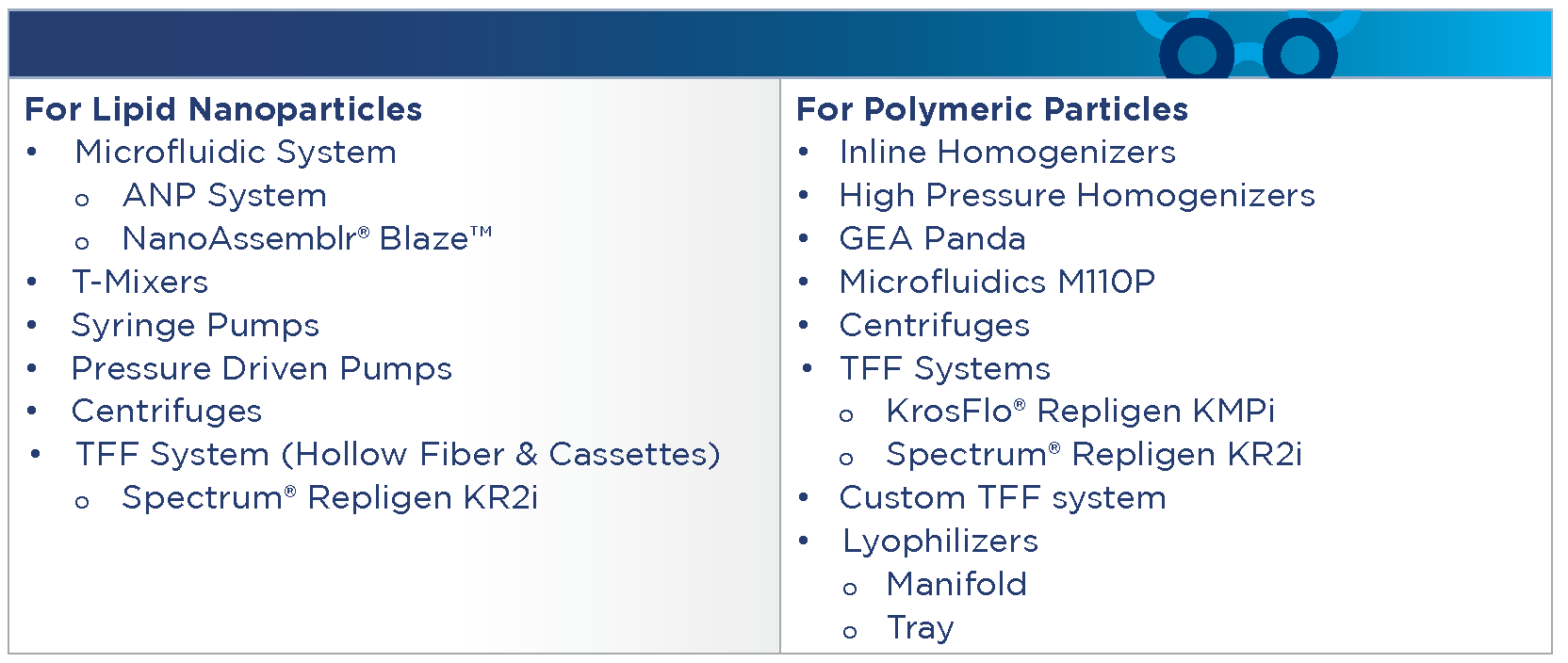
For Process Development, we offer the following systems, equipment, and technologies:
Our Analytical Services
At Phosphorex, our analytical services team is dedicated to the meticulous characterization of particulate-based drug delivery systems. Our expertise spans the entire pharmaceutical development journey, from early feasibility studies to clinical trials. Click here to find out more about our Analytical Services.
Interested in finding our more about our PD & Scale-up services, email us at bd@phosphorex.com